|
|
Molecule of the Month August 2003 |
|
| Dihydroxyacetone (Dha) in Hemiaminal Linkage to the Active Site of Dihydroxyacetone Kinase (Swiss-Prot P76015) | ||
|
||
|
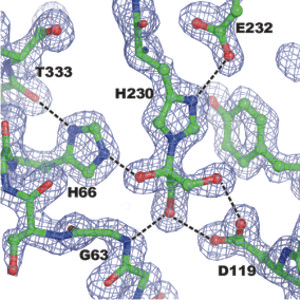
Dihydroxyacetone (Dha) in hemiaminal linkage to the active site of dihydroxyacetone kinase (Swiss-Prot P76015). The hemiaminal is formed between the carbonyl carbon of Dha and the imidazole nitrogen of histidin-230 (HOCH3-COH(-NHis)-CH3OH). The substrate is further stabilized by a network of hydrogen bonds. The covalent bond does not participate in catalysis but is a means to discriminate between the chemically reactive Dha and the structurally similar but inert glycerol.
This work was carried out in the groups of Prof. Bernhard Erni / Prof. Ulrich Baumann. References:
|
||