|
|
Molecule of the Month December 2003 |
|
| Crystal Structure of the Citrobacter freundii Dihydroxyacetone Kinase Reveals an Eight-stranded a-Helical Barrel ATP-binding Domain | ||
|
||
|
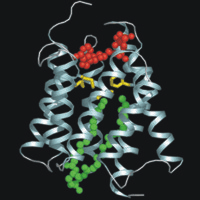
This is the nucleotide binding domain of the dihydroxyacetone kinase of Citrobacter freundii complexed with AMPPNP (red) and a phospholipid (green). Met and Phe (yellow) separate the nucleotide-binding depression from the lipid-binding pocket of the eight-helix barrel. Homologous domains occur in the PTS-dependent kinase of E.coli and proteins associated with various transcription factors. This work was carried out in the group of Prof. Bernhard Erni. References:
|
||