|
|
Molecule of the Month May 2011 |
|
| Competitive Anion Interactions at Copper/Electrolyte Interfaces | ||
|
||
|
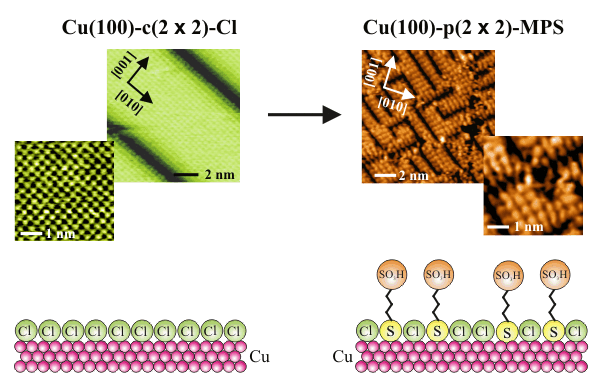
The time-dependent, competitive interaction between chloride anions and SPS (Bis-(3-sulfopropyl)-disulfid) for adsorption sites on copper/electrolyte interfaces is of central importance for the so-called Copper Damascene Process. Copper electrodeposition is used in today's microprocessor fabrication for the metallization of interconnects on Si-chips. The diameter of those copper interconnects currently approaches the sub-50nm regime (22nm and 16nm technology).
This work was carried out in the group of PD Dr. Peter Broekmann. References:
|
||