|
|
Molecule of the Month September 2011 |
|
| Redox-Active Nanographene | ||
|
||
|
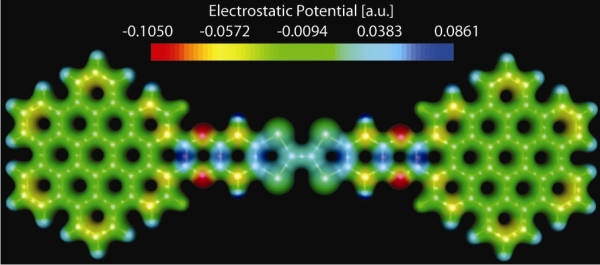
An electrochemically highly amphoteric nanographene was synthesized and characterized experimentally and theoretically. The synthetic strategy involves the attainment of a tetrathiafulvalene (TTF) derivative with two diamine moieties which also plays a crucial role in the development of diverse TTF-based functional materials.
This work was carried out by Dr. Shi-Xia Liu et al. in the group of Prof. Silvio Decurtins. References:
|
||