|
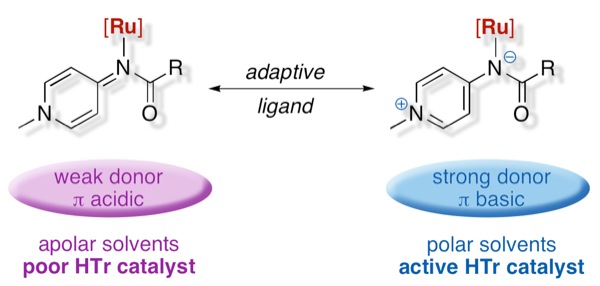
Pyridylideneamides are easily accessible ligands that adapt to their environment as they can switch between a formally anionic and a neutral donor state.
Low metal oxidation states emphasize the neutral π-acidic form (picture left), while high-valent metals are stabilized by the zwitterionic state and thus increase the ligand π-donor properties (right). This ligand flexibility has implications in redox catalysis and provides a new approach to enhance catalytic performance.
This work was carried out in the group of Prof. Martin Albrecht.
References:
-
K. F. Donnelly, C. Segarra, L.-X. Shao, R. Suen, H. Müller-Bunz, M. Albrecht;
"Adaptive N-Mesoionic Ligands Anchored to a Triazolylidene for Ruthenium-Mediated (De)Hydrogenation Catalysis"
Organometallics, 2015, 34(16), 4076-4084;
doi:10.1021/acs.organomet.5b00533.
-
V. Leigh, D. J. Carleton, J. Olguin, H. Müller-Bunz, L. J. Wright, M. Albrecht;
"Solvent-Dependent Switch of Ligand Donor Ability and Catalytic Activity of Ruthenium(II) Complexes Containing Pyridinylidene Amide (PYA) N-Heterocyclic Carbene Hybrid Ligands"
Inorg. Chem., 2014, 53(15), 8054-8060;
doi:10.1021/ic501026k.
|