|
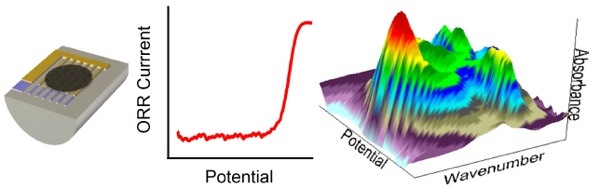
Electrocatalysis - the field of catalysis where the catalytic process involves a charge transfer - is crucial for establishing a sustainable energy supply. To develop improved electrocatalysts, we need to understand the electrocatalysts under reaction conditions. For this purpose we developed an in-situ FTIR spectroscopy setup that allows us to study industrial-type electrocatalysts under defined mass transport conditions during reactions [1].
In our recent work [2] we used this setup to show that the fuel cell cathode reaction, oxygen reduction reaction (ORR), is controlled by anion adsorption and the oxidation of the Pt catalyst. In contrast to previous models, however, we observe that the anion coverage decreases with the onset of the ORR inhibition.
This work was carried out in the group of Prof. Matthias Arenz.
References:
-
M. Nesselberger, S. J. Ashton, G. K. H. Wiberg, M. Arenz;
"Design, development, and demonstration of a fully LabVIEW controlled in situ electrochemical Fourier transform infrared setup combined with a wall-jet electrode to investigate the electrochemical interface of nanoparticulate electrocatalysts under reaction conditions"
Rev. Sci. Instrum., 2013, 84(7), 074103-074107;
doi:10.1063/1.4816826.
-
M. Nesselberger, M. Arenz;
"In Situ FTIR Spectroscopy: Probing the Electrochemical Interface during the Oxygen Reduction Reaction on a Commercial Platinum High-Surface-Area Catalyst"
ChemCatChem, 2016, 8(6), 1125-1131;
doi:10.1002/cctc.201501193.
|