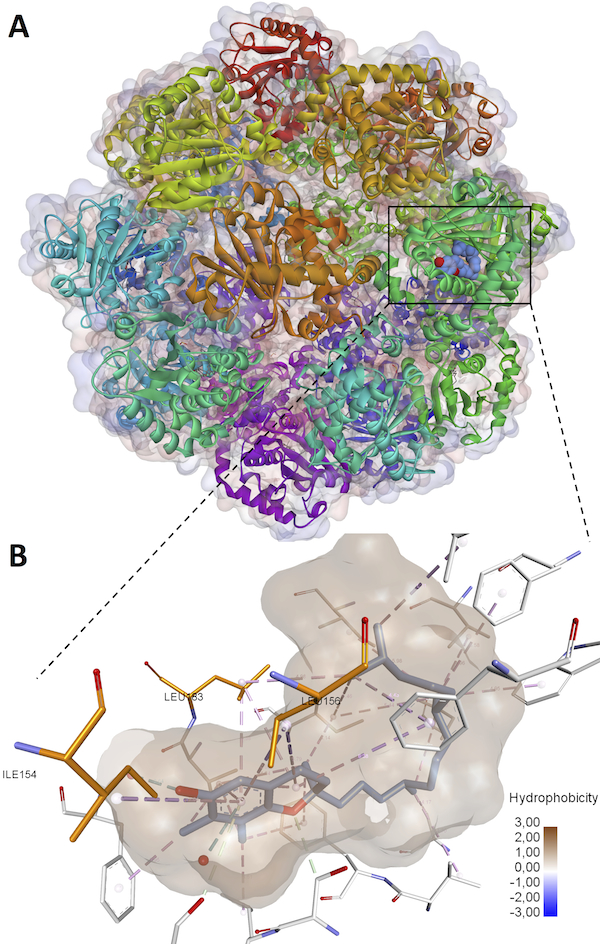
|
α-Tocopherol Transfer Protein (α-TTP) sequesters α-tocopherol out of eight naturally occurring Vitamin E isomers in man [1]. The reasons for the preferential α-tocopherol homeostasis over the similarly abundant γ-tocopherol isomer are currently not known.
In this work, we describe the engineering of a biophysically functional TTP variant with its vitamer selectivity shifted towards γ-tocopherol [2]. Using primary human umbilical vein cells (HUVEC) it is shown that α-TTP significantly reduces cytokine-induced mRNA expression of the pro-inflammatory cytokines IL6, CCL2, TNF and ICAM1 while γ-TTP is not able to attenuate cytokine-mediated transcription. The observed differences indicate that cytokine suppression occurs primarily in the presence of α-tocopherol and also provide clues on the preferential homeostasis of the later in man.
This work was carried out in the group of Prof. Achim Stocker.
References:
-
K. Ouahchi, M. Arita, H. Kayden, F. Hentati, M. B. Hamida, R. Sokol, H. Arai, K. Inoue, J.-L. Mandel, M. Koenig;
"Ataxia with isolated vitamin E deficiency is caused by mutations in the α-tocopherol transfer protein"
Nature Genetics, 1995, 9(2), 141-145;
doi:10.1038/ng0295-141.
-
W. Aeschimann, S. Kammer, S. Staats, P. Schneider, G. Schneider, G. Rimbach, M. Cascella, A. Stocker;
"Engineering of a functional γ-tocopherol transfer protein"
Redox Biology, 2021, 38, 101773/1-12;
doi:10.1016/j.redox.2020.101773.
|