|
|
Molecule of the Month February 2023 |
|
| Predicting the Zoonotic Potential of Primate Erythroparvoviruses | ||
|
||
|
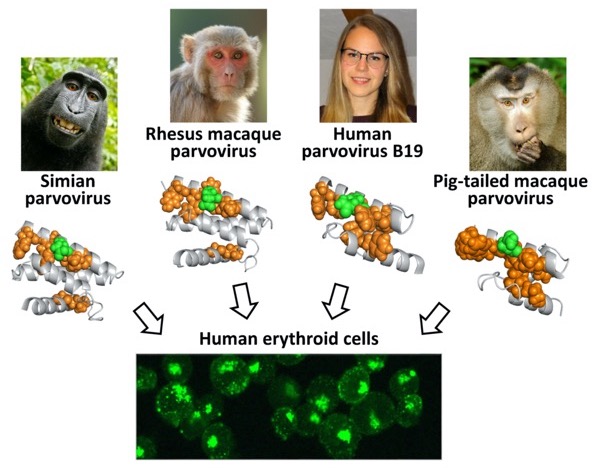
Sequence alignment and in silico protein structure prediction of the receptor-binding domain (RBD) from human and other primate erythroparvoviruses (simian, rhesus, and pig-tailed) revealed a similar structure characterized by a fold of three or four α-helices.
This work was carried out in the group of PD Dr. Carlos Ros. References:
|
||