|
|
Molecule of the Month July 2009 |
|
| Persistent Radical Meets Boron Enolates | ||
|
||
|
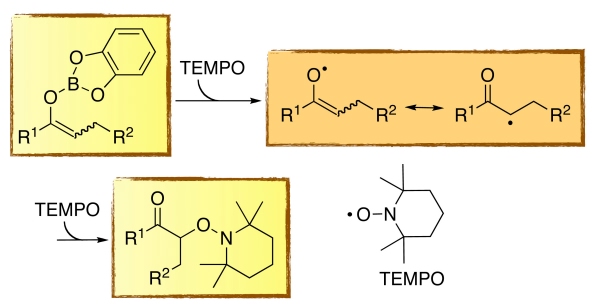
Mild and efficient oxidation of catecholboron ketone enolates is performed by reaction with the persistent TEMPO radical. Catecholboron enolates are readily prepared via 1,4-reduction of α,β-unsaturated ketones, via "transmetallation" of silyl enol ethers and zinc enolates.
This work was carried out in the group of Prof. Philippe Renaud. References:
|
||