|
|
Molecule of the Month September 2009 |
|
| de-novo Identification of a Complex Disulfide Bridge Pattern | ||
|
||
|
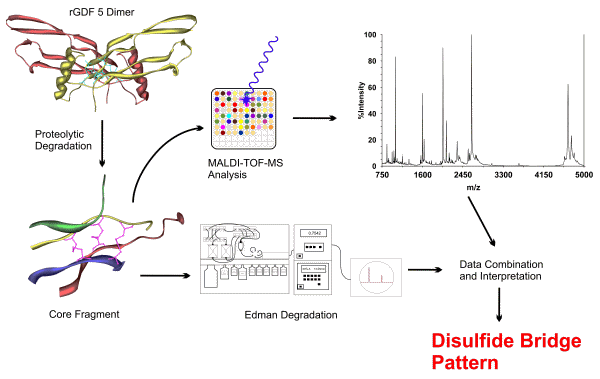
The cystine knot structure of homodimeric recombinant human growth and differentiation factor 5 (rGDF5) was identified de novo applying a combination of proteolytic degradation and subsequent analyses of the disulfide-linked peptides by electrospray- and MALDI-TOF mass spectrometry, amino acid analysis, and Edman degradation.
This work was carried out in the groups of PD Dr. Stefan Schürch and Prof. Johann Schaller. References:
|
||