|
Mitomycin alkaloids are an important class of bioactive natural products having potent antibiotic and antitumor properties.
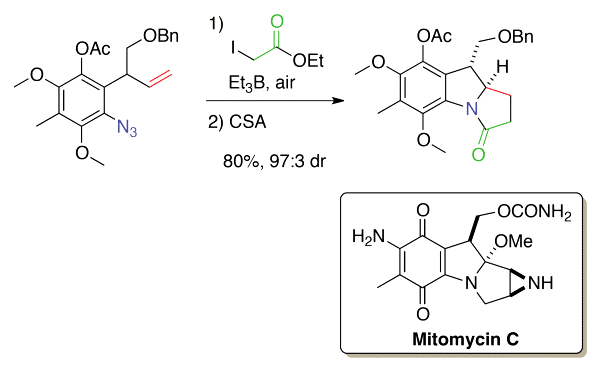
By an efficient diastereoselective radical cascade/lactamisation sequence, a mitomycin derivative has been prepared from a highly functionalised ortho-azidoallylbenzene intermediate using triethylborane to initiate and sustain the radical step.
This work was carried out in the group of Prof. Philippe Renaud.
References:
-
F. Brucelle, P. Renaud;
"Synthesis of a Leucomitosane via a Diastereoselective Radical Cascade"
J. Org. Chem., 78, 6245-6252, (2013);
doi:10.1021/jo4009904.
-
F. Brucelle, P. Renaud;
"Synthesis of Indolines, Indoles, and Benzopyrrolizidinones from Simple Aryl Azides"
Org. Lett., 14, 3048-3051, (2012);
doi:10.1021/ol301120w.
|