|
|
Molecule of the Month March 2018 |
|
| Radical Deuteration with D2O | ||
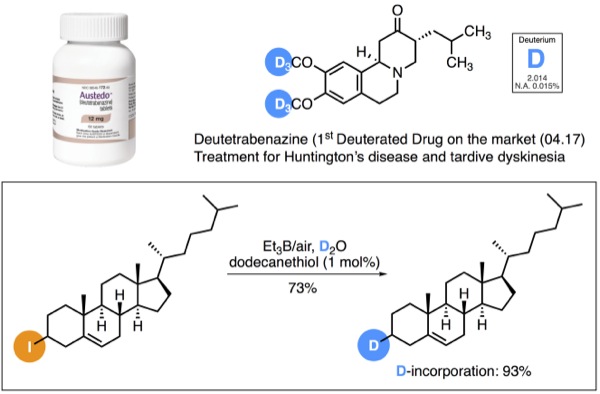
|
||
|
Recently, there has been a growing interest in the pharmaceutical industries to incorporate deuterium in drugs candidates to improve their metabolism and pharmacokinetic properties such as Deutetrabenazine (Austedo®). [1]
This work was carried out in the group of Prof. Dr. Philippe Renaud. References:
|
||