|
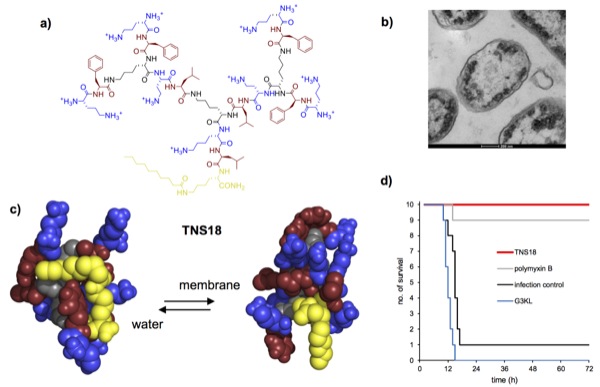
New antibiotics are urgently needed to address multidrug resistant (MDR) bacteria. Herein we report that second generation (G2) peptide dendrimers bearing a fatty acid chain at the dendrimer core such as TNS18 (structure shown in a) efficiently kill Gram-negative bacteria including Pseudomonas aeruginosa and Acinetobacter baumannii, two of the most problematic MDR bacteria worldwide, by a membrane disruptive mechanism (b).
Based on circular dichroism and molecular dynamics studies we hypothesize that TNS18 adopts a hydrophobically collapsed conformation in water with the fatty acid chain backfolded onto the peptide dendrimer branches, and that the dendrimer unfolds in contact with the membrane to expose its lipid chain and hydrophobic residues, thereby facilitating membrane disruption leading to rapid bacterial cell death (c). Dendrimer TNS18 shows promising in vivo activity against MDR clinical isolates of A. baumannii (d) and E. coli, suggesting that lipidated peptide dendrimers might become a new class of antibacterial agents.
This work was carried out in the group of Prof. Dr. Jean-Louis Reymond.
References:
-
T. N. Siriwardena, M. Stach, R. He, B.-H. Gan, S. Javor, M. Heitz, L. Ma, X. Cai, P. Chen, D. Wei, H. Li, J. Ma, T. Köhler, C. van Delden, T. Darbre, J.-L. Reymond;
"Lipidated Peptide Dendrimers Killing Multidrug-Resistant Bacteria"
J. Am. Chem. Soc., 2018, 140(1), 423-432;
doi:10.1021/jacs.7b11037.
|