|
|
Molecule of the Month May 2020 |
|
| Structure and Mechanism of the ER-Based Glucosyltransferase ALG6 | ||
|
||
|
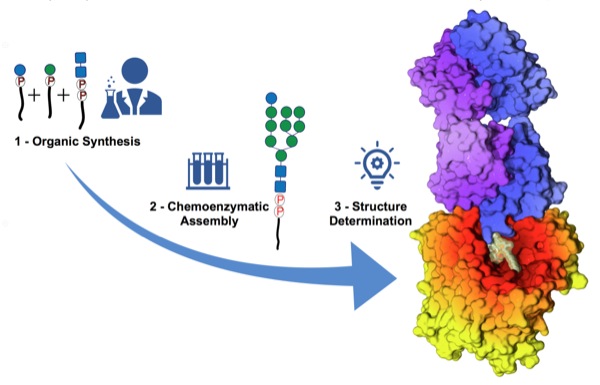
In eukarya protein N-glycosylation, a series of reactions occurs within the endoplasmic reticulum (ER) where a dolichylpyrophosphate-linked oligosaccharide is assembled stepwise. We used synthetic analogues of dolichylphosphate-linked and dolichylpyrophosphate-linked sugars and purified enzymes of the ALG pathway to elongate the glycan and recapitulate the activity of ALG6 in vitro.
This work was carried out in the group of Prof. Jean-Louis Reymond. Reference:
|
||