|
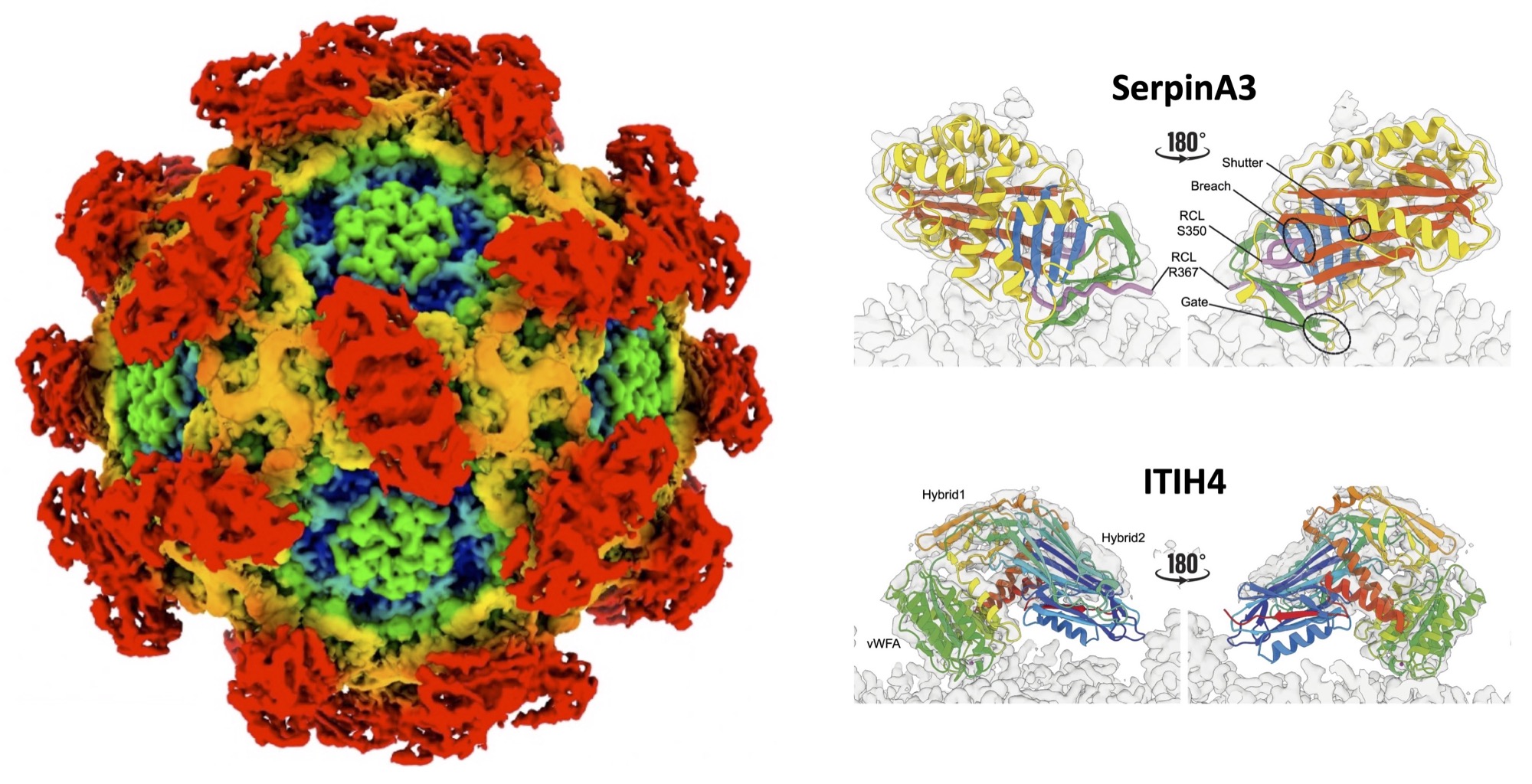
The 2.2 Å structure of B19V from patient blood revealed two host protease inhibitors, ITIH4 and serpinA3, bound at distinct capsid sites. These protease inhibitors remain active and dissociate before cell entry, suggesting a novel shielding mechanism for immune evasion and protease resistance.
This discovery, made by studying authentic virions from infected patients rather than the commonly used cell culture-derived particles, suggests that other viruses may exploit similar strategies to enhance infectivity.
This work was carried out in the group of PD Dr. Carlos Ros.
References:
-
H. Lee, R. Assaraf, S. Subramanian, D. Goetschius, J. Bieri, N. M. DiNunno, R. Leisi, C. M. Bator, S. L. Hafenstein, C. Ros;
"Infectious parvovirus B19 circulates in the blood coated with active host protease inhibitors"
Nature Communications, 2024, 15, 9543;
doi:10.1038/s41467-024-53794-1.
-
This week in virology (TWiV); November 17, 2024;
https://www.microbe.tv/twiv/twiv-1167/.
-
Horizons - The Swiss Research Magazine; March 6, 2025;
https://www.horizons-mag.ch/2025/03/06/an-invisibility-cloak-for-a-virus/.
|