|
|
Molecule of the Month April 2004 |
|
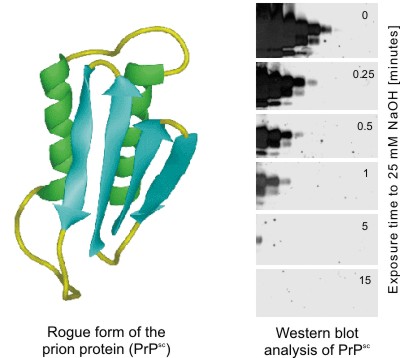
| Sodium Hydroxide Renders the Prion Protein PrPsc Sensitive to Proteinase K | ||
|
||
|
The partially proteinase resistant form of the prion protein (PrPsc) is thought to be the causative agent of transmissible spongiform encephalopathies (TSE, e.g. BSE). PrPsc is one of the most stable proteins known. According to WHO recommendation inactivation of this protein requires incubation with 2M NaOH for > 1hr.
This work was carried out in the group of Prof. Christoph Kempf. References:
|
||