|
Molecular crystals studied under high pressure reveal features of the molecular potential energy surface.
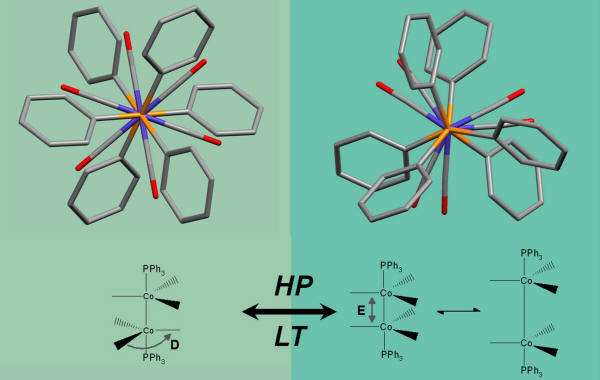
In the picture, an example of what happens to species Co2(CO)6(XPh3)2 (X = As, P) at high pressure (HP) or low temperature (LT). Investigations are carried out using experimental X-ray diffraction and ab initio calculations (in the gas and in the solid state phase).
The external pressure induces a change of the molecular conformation (carbonyls are staggered at room conditions, but almost eclipsed above 4.0 GPa) and consequently a lengthening of the Co-Co bond (because of the enhanced intra-carbonyl repulsion).
This work was carried out in the recently established Chemical Crystallography Group at the Department of Chemistry and Biochemistry (Dr. P. Macchi), in collaboration with Dr. N. Casati and Prof. A. Sironi (University of Milan).
References:
-
N. Casati, P. Macchi and A. Sironi;
"Staggered to Eclipsed Conformational Rearrangement of [Co2(CO)6(PPh3)2] in the Solid State: An X-ray Diffraction Study at High Pressure and Low Temperature"
Angew. Chem. Int. Ed., 44, 7736-7739, (2005);
doi:10.1002/anie.200502241.
-
N. Casati, P. Macchi and A. Sironi;
"Molecular Crystals Under High Pressure: Theoretical and Experimental Investigations of the Solid-Solid Phase Transitions in [Co2(CO)6(XPh3)2] (X=P, As)"
Chem. Eur. J., 15, 4446-4457, (2009);
doi:10.1002/chem.200801528.
|